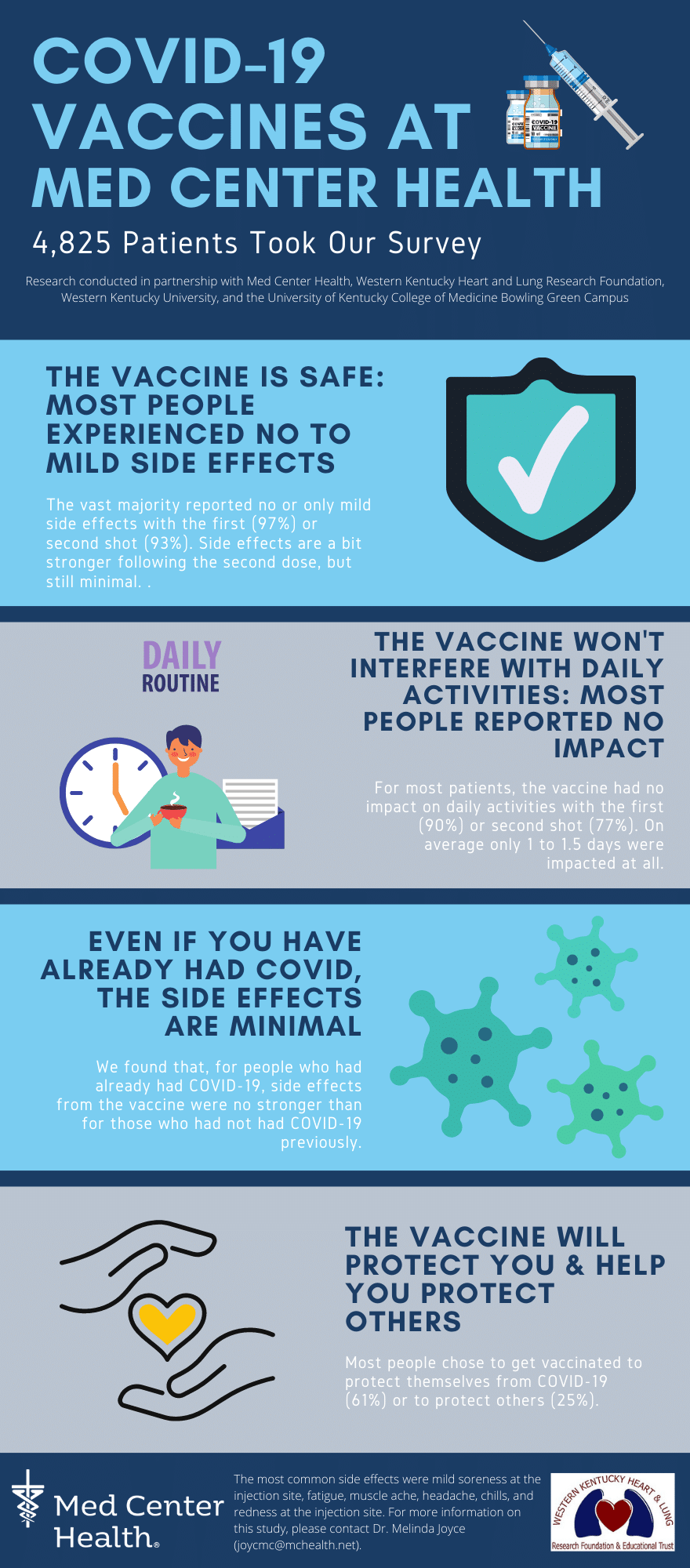
Local Research Study Confirms COVID-19 Vaccine Safety
A collaborative research project between Med Center Health, Western Kentucky University and the UK College of Medicine – Bowling Green Campus was conducted through the Western Kentucky Heart and Lung/Med Center Health Research Foundation. The hypothesis of the research centered around responses to the COVID-19 vaccine – specifically, whether or not responses seen in an ambulatory vaccine clinic setting were different than what was seen in the clinical trials. The results from 4,825 patients who took the survey mirror the results in the COVID-19 clinical trials.
Results of the survey were:
- The vaccine was safe and most people experienced either no symptoms or mild symptoms
- Individuals receiving Moderna had more symptoms than the individuals receiving Pfizer, but still were in the mild category
- Most people reported the side effects did not interfere with their daily activities and if they did, that interference lasted about 1 to 1.5 days
- People who were previously COVID positive prior to vaccination tended to have more side effects with the first doses, but the side effects were still minimal
- People overwhelmingly received the vaccine to protect themselves and others
Qualtrics was used as the survey tool, which allowed participants to answer on their phone or by computer. The participants had received at least one dose of vaccine from Med Center Health (all MCH locations). A total of 17,760 texts were initially sent on May 5. Text reminders were sent on May 12 and June 12. On June 12, another 1,830 individuals were added for a total of 19,590 potential respondents. The survey closed on June 21. Some of the questions included:
- Demographic information
- Why did you choose to get vaccinated?
- What brand of vaccine did you receive?
- Were you previously COVID positive?
- For various side effects, participants rated their response after the first dose and again after the second dose. Participants were able to enter another type of reaction if it was not listed.
- Did you miss any work or other activity due to side effects; and, if so, how long did that last?
- Did you need to take a medication, such as acetaminophen or ibuprofen to manage the side effects?
- Participants were asked to rate their reactions to other vaccines in a similar rating scale to what was asked for the COVID vaccine.
In summary, the research survey found most people experienced mild side effects, the vaccine did not interfere with daily activities, and side effects for people who had already been diagnosed with COVID-19 were minimal. The majority of people chose to be vaccinated to protect themselves and others.
“Although our findings were no different than those of the clinical trials,” said Melinda Joyce, PharmD, FAPhA, FACHE, principal investigator for the research project, “conducting a robust research project in Southcentral Kentucky regarding responses to the COVID-19 vaccines by people who received the vaccine in this area is beneficial. The findings of this study further illustrate the safety of the COVID-19 vaccine for our community.”
The most common side effects to the COVID-19 vaccine were mild soreness at the injection site, fatigue, muscle ache, headache, chills and redness at the injection site. For more information on the research study, contact Melinda Joyce, PharmD, FAPhA, FACHE, Executive Director of the Western Kentucky Heart and Lung/Med Center Health Research Foundation, at JoycMC@MCHealth.net.
(Click image to enlarge)